

传真:0755-81484129
邮箱:shi145320@126.com
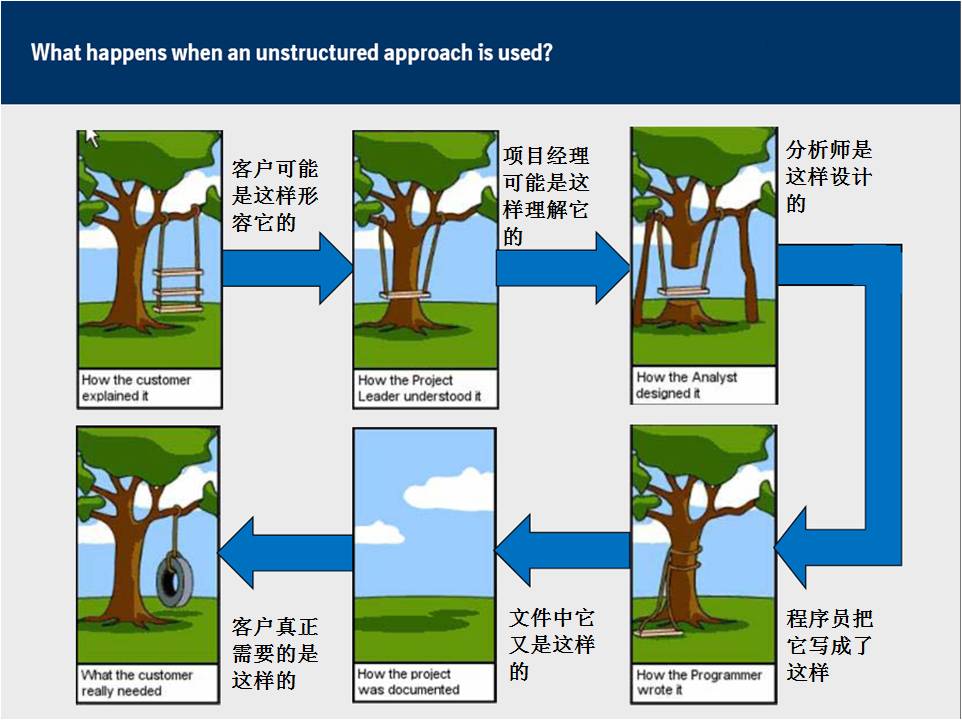
一个管理不善的项目可能是这样的:
因此,引进一台设备/系统或者新建厂房时URSFSDSFATSATDQIQOQPQ之间是环环相扣的。然而这些制药行业常见的术语很多人并不清楚。本文将对这些术语的含义、关系以及引进一个设备时它们的顺序进行阐述。
URS(User requirement specifications)
用户需求规范
Manufacturers should prepare a document that describes, for example, the utility or equipment to be sourced. The requirements and specifications for the utility or equipment should be defined by the user and documented in the URS.
生产者应该准备一个文件描述,如需要采购的设施或设备。设施或设备的需求和规范应该被用户定义并写入在URS文件中。
The URS should be used when selecting the required utility or equipment from an approved supplier, and to verify suitability throughout the subsequent stages of qualification.
URS应该在从批准的供应商中选择设施或设备时被使用,并在随后的确认的各个阶段用于证实适用性。
FAT和SAT(Factory acceptance test and site acceptance test)
工厂验收测试和现场验收测试
Where appropriate, FAT and SAT should be performed to verify the suitability of the system at site, prior to the subsequent stages of qualification. This should be appropriately documented.
在适当情况下,在随后的确认阶段之前,FAT和SAT应该被执行来证实系统在现场的适用性。这应该被适当的记录。
(FAT,Factory acceptance test ,这个名称原来并非一个GMP的名词,而是GEP的概念,但是在新的欧盟GMP附录15和修订的WHO GMP 验证指南中,已经增加了这个内容,现在是GMP的要求了,这也算是质量向前延伸吧。工厂验收测试,顾名思义,在工厂验收时做的测试,这个工厂,并非是使用者,而是设备制造商。为什么需要做这个测试呢?很简单,因为有些项目,在设备制造后,你是无法再对其进行测试的,但是如果到公司了,你才发现不合格,可能就晚了,比如说,反应罐,你到公司了,才发现容量不对,你要再换一个,浪费人力物力。所以需要你在设备出厂前,就在工厂进行测试,确保设备到工厂前,就是合格的。FAT的方案一般会有设备厂家提供,使用方审核,如果使用方也可以根据自己的要求,起草方案,供应商确认。FAT的内容会和IOQ有些重复,两者之间是否能够代替,各有各的说法,我个人认为如果有些项目,FAT中做了测试,而运输过程对这些又不会产生影响,在IQ中,是可以引用FAT的内容,而不需要进行重复测试,如软件的计算功能。
SAT,Site Acceptance
Testing,现场验收测试,同样,这个名词最初是GEP中的概念,在新的欧盟GMP附录15和修订的WHO GMP
验证指南中,也增加了这个内容。SAT和FAT相对应,FAT是在设备制造商处进行的测试,SAT就是在使用者这里进行的测试了。那么SAT和IOQ之间有什么区别和联系呢?说实在的,我的理解是两者之间,差别不大,如果硬要说有差别,SAT的测试范围可能更为广泛,包括一些在安装调试过程中,进行的一些额外的测试。IOQ可能就是只需要确认最后的安装状态就好。个人认为,如果SAT做的详细,IOQ文件完全可以引用SAT,当然,前提条件是,足够详细,能够完全涵盖到IOQ的测试内容。同样,一般SAT的方案,好的设备供应商有较为详细的方案,这个方案是需要使用者审核确认的,使用者也可以根据自己的要求,提出SAT方案。)
IQ(Installation qualification)
安装确认
IQ should provide documented evidence that the installation was complete and satisfactory.
IQ应该提供文件证据证实安装已完成并且是令人满意的。
The design specifications, including purchase specifications, drawings, manuals, spare parts lists and vendor details should be verified during IQ as should the configuration specifications for the intended operational environment.
设计规范,包括采购规范、图纸、说明书,备件清单和供应商详情应该在IQ阶段被核实。预期的操作环境的配置规范也一样。
Components installed should be verified and documented evidence should be provided that components meet specifications, are traceable and are of the appropriate material of construction.
部件安装应该被核实并且应提供文件依据证实部件符合规范要求,可追溯并由适当的材质组成。
Control and measuring devices should be calibrated.
控制和测量装置应该被校准。
OQ(Operational qualification)
运行确认
OQ should provide documented evidence that utilities, systems or equipment and all its components operate in accordance with operational specifications.
OQ应该提供文件证据证实设施、系统或设备以及它的所有部件的运转符合操作说明书。
Tests should be designed to demonstrate satisfactory operation over the normal operating range as well as at the limits of its operating conditions (including worst-case conditions).
测试应该被设计来证实在正常操作范围和操作条件的边界(包括最差条件)下满意的运行。
Operation controls, alarms, switches, displays and other operational components should be tested.
运行控制、报警、开关、显示和其它操作部件应该被测试。
Measurements made in accordance with a statistical approach should be fully described.
应该充分描述按照统计学方法设计的测量。
PQ:Performance qualification
性能确认
P Q should be conducted prior to release of the utilities, systems or equipment under conditions simulating conditions of intended use to provide documented evidence that utilities, systems or equipment and all its components can consistently perform in accordance with the specifications under routine use.
PQ应该在设施、系统或设备放行前执行模拟预期使用的条件来提供文件证据证实设施、系统或设备以及它的所有部件在日常使用下可以一贯地符合标准。
Test results should also be collected over a suitable period of time during continuous process verification and/or periodic review and monitoring of the utilities, systems and equipment to prove consistency.
测试结果还应在连续工艺核实期间和/或周期回顾和监测的适当时期收集来证实一致性。
它们的顺序:
Stages of qualification should normally start with the preparation of user requirement specifications (URS). Depending on the function and operation of the utility, equipment or system, this is followed by, as appropriate, different stages in qualification such as a factory acceptance test (FAT), site acceptance test (SAT), design qualification (DQ), installation qualification (IQ), operational qualification (OQ) and performance qualification (PQ).
通常地,确认应该从用户需求规范(URS)的准备开始。然后根据设施、设备或者系统的功能和操作特点,选择不同的确认阶段,如工厂验收测试(FAT),现场验收测试(SAT),设计确认(DQ)、安装确认(IQ)、运行确认(OQ)和性能确认(PQ)。
One stage of qualification should be successfully completed before the next stage is initiated, e.g. from IQ to OQ.
确认时,一个阶段在开始下一个阶段前被成功地完成,例如,从IQ到OQ。
In some cases, only IQ and OQ may be required, as the correct operation of the equipment, utility or system could be considered to be a sufficient indicator of its performance.
在某些情况下,只需要进行IQ和OQ,设备、设施或系统的正确操作就可以被认为它的性能的一个充分的指标了。
Major equipment and critical utilities and systems, however, may require URS, DQ, IQ, OQ and PQ.
主要设备和关键设施和系统则可能需要URS、DQ、IQ、OQ和PQ。
Computerized systems, including equipment with software component(s), require user and functional requirements specifications, design and configuration specifications, development of SOPs, training programmes for system use and administration, and an appropriate level of IQ, OQ and PQ verification testing. This includes tests such as stress, load, volume and other performance verification tests that mimic the live production environment. It also includes user acceptance testing according to draft SOPs and training as well as end-to-end business processes for intended use.
计算机化系统,包括有软件构件的设备,需要用户和功能需求规范、设计和配置规范,开发SOP,系统使用和管理的培训计划,和一个适当水平的IQ、OQ和PQ测试。这包括测试如压力、负载、容量和其他模拟动态生产环境的性能测试。这也包括根据SOP草案和培训以及对预期使用的业务流程的用户验收测试。
它们的关系:
FAT、SAT没有交叉
FAT、IQ、OQ可能有交叉
IQ和OQ也没有交叉
SAT和IQ/OQ可能有交叉
